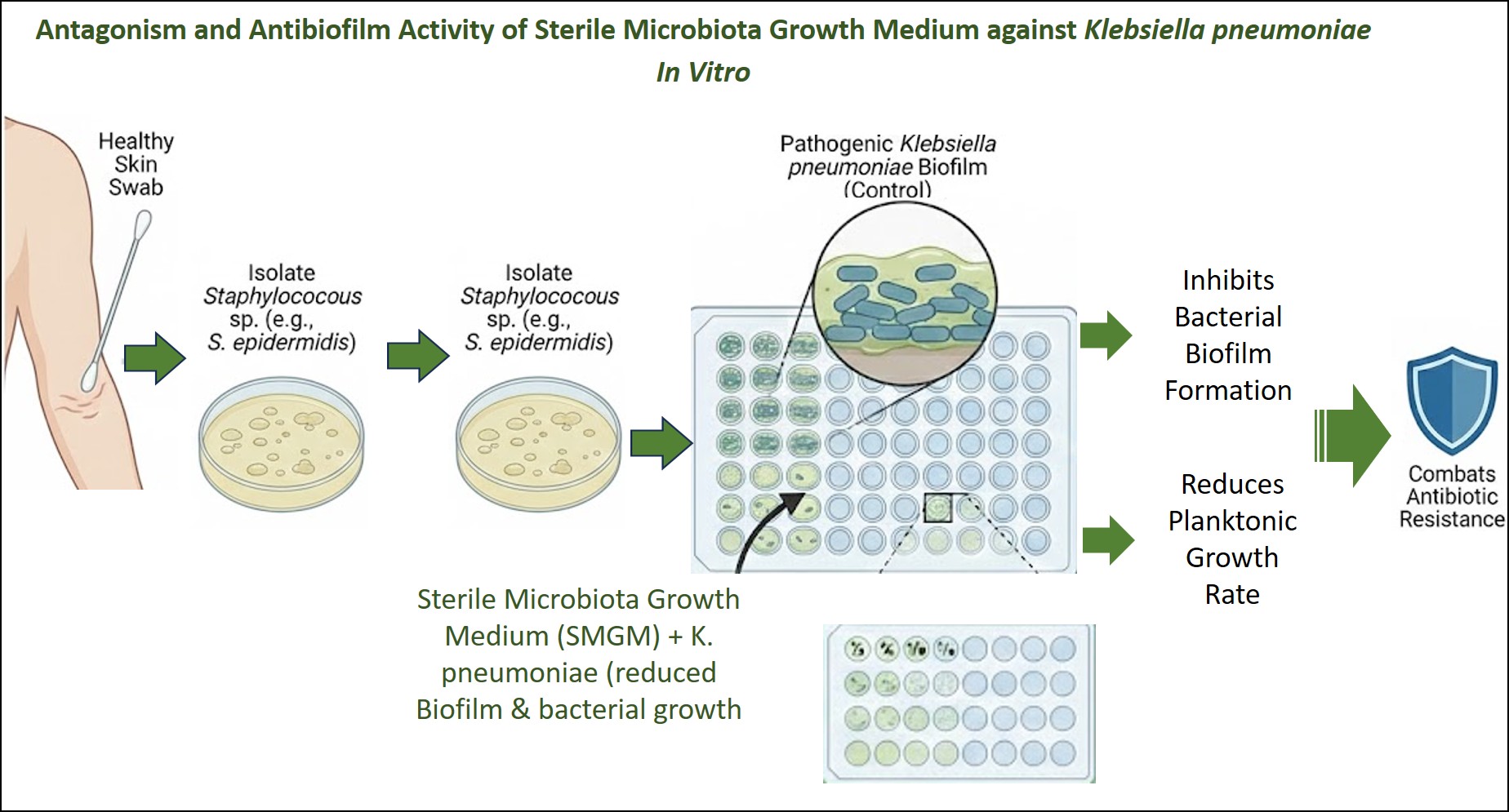
Antagonism and Antibiofilm Activity of Sterile Microbiota Growth Medium against Klebsiella pneumoniae In Vitro
DOI:
https://doi.org/10.65329/wjeb.v13.02.003Keywords:
Antibiofilm, Antagonism, Biofilm, Klebsiella pneumoniae, MicrobiomeAbstract
The metabolic bacteria extraction, Sterile Microbiota Growth Medium (SMGM), may play a role as an antibacterial and antibiofilm agent, reducing the virulence of pathogenic bacteria in vitro and in vivo. These extracts can be used to treat infections caused by antibiotic-resistant bacterial isolates. The present study aims to investigate the antibacterial and antibiofilm effects of SMGM extracted from non-Staphylococcus aureus growth media on the virulence of Klebsiella pneumoniae, as assessed by biofilm formation and bacterial growth rate. In the current study, 25 skin swabs were collected from healthy volunteers to isolate non-S. aureus isolates (S. epidermidis). From these isolates, SMGMs were prepared aseptically from 10 isolates of S. epidermidis by collecting the overnight growth (Nutrient broth) and preparing cell-free growth media after centrifugation and passing through Millipore filters. The microdilution method on a microtiter plate was used to evaluate the antibacterial effect of SMGM. Moreover, the microtiter plate and crystal violate method was used to assess the antibiofilm effect of different dilution of SMGM on the ability of K. pneumoniea to form biofilm in vitro. The results showed that all dilutions of SMGM ½, ¼, 1/8, 1/16, 1/32, and 1/64 reduced biofilm formation of K. pneumoniae (P<0.05). The study also demonstrated that SMGM reduced the growth rate of K. pneumoniae (P<0.05) at different time intervals (up to 48 h). It can be concluded from the current study that SMGM reduces the ability of K. pneumoniae to form biofilms and decreases the growth rate of planktonic cells.
References
[1] Liu Y, Huang L, Cai J, Zhu H, Li J, et al. (2023) Clinical characteristics of respiratory tract infection caused by Klebsiella pneumoniae in immunocompromised patients: a retrospective cohort study. Front Cell Infect Microbiol 13:1137664. http://doi.org/10.3389/fcimb.2023.1137664. PMID: 37662019; PMCID: PMC10469001.
[2] Song S, Yang S, Zheng R, Yin D, Cao Y, et al. (2024) Adaptive evolution of carbapenem-resistant hypervirulent Klebsiella pneumoniae in the urinary tract of a single patient. Proc Natl Acad Sci U S A 121(35):e2400446121. http://doi.org/10.1073/pnas.2400446121. PMID: 39150777; PMCID: PMC11363291 .
[3] Guerra MES, Destro G, Vieira B, Lima AS, Ferraz LFC, et al. (2022) Klebsiella pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front Cell Infect Microbiol. 12:877995. doi.org/10.3389/fcimb.2022.877995. Erratum in: Front Cell Infect Microbiol 15:1564010. http://doi.org/10.3389/fcimb.2025.1564010. PMID: 35646720; PMCID: PMC9132050.
[4] Assefa M, Amare A. (2022) Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. Infect Drug Resist 15:5061-5068. http://doi.org/10.2147/IDR.S379502. PMID: 36068834; PMCID: PMC9441148 .
[5] Dan B, Dai H, Zhou D, Tong H, Zhu M. (2023) Relationship Between Drug Resistance Characteristics and Biofilm Formation in Klebsiella Pneumoniae Strains. Infect Drug Resist 16:985-998. http://doi.org/10.2147/IDR.S396609. PMID: 36824066; PMCID: PMC9942501 .
[6] Le VH, King T, Wuerzberger B, Bauer OR, Carver MN, et al. (2025) Metabolites derived from bacterial isolates of the human skin microbiome inhibit Staphylococcus aureus biofilm formation. Microbiol Spectr 5:e0130625. http://doi.org/10.1128/spectrum.01306-25. PMID: 40762463.
[7] Ali MN, Heydarlou MM. (2023) Histopathological Effect of Extracellular Products of Clinical Isolates of Pseudomonas aeruginosa on Mice Lungs. World J Exp Biosci 11(2): 36-40. http://doi.org/10.65329/wjeb.v11.02.003
[8] Xu Q, Hu X, Wang Y. (2021) Alternatives to Conventional Antibiotic Therapy: Potential Therapeutic Strategies of Combating Antimicrobial-Resistance and Biofilm-Related Infections. Mol Biotechnol 63(12):1103-1124. http://doi.org/10.1007/s12033-021-00371-2. PMID: 34309796.
[9] Watanabe N, Watari T, Otsuka Y, Yamagata K, Fujioka M. (2022) Clinical characteristics and antimicrobial susceptibility of Klebsiella pneumoniae, Klebsiella variicola and Klebsiella quasipneumoniae isolated from human urine in Japan. J Med Microbiol 71(6). http://doi.org/10.1099/jmm.0.001546. PMID: 35699119.
[10] Al-Mutalib LAA Zgair AK (2023) Sub-inhibitory doses of Ofloxacin reduce adhesion and biofilm formation of Pseudomonas aeruginosa to biotic and abiotic surfaces. Pharm Sci Asia 50(3): 196-203. http://doi.org/10.29090/psa.2023.03.23.377 .
[11] Ratajczak M, Kaminska D, Dlugaszewska J, Gajecka M. (2021) Antibiotic Resistance, Biofilm Formation, and Presence of Genes Encoding Virulence Factors in Strains Isolated from the Pharmaceutical Production Environment. Pathogens 10(2):130. http://doi.org/10.3390/pathogens10020130. PMID: 33513933; PMCID: PMC7911615.
[12] Li Y, Kumar S, Zhang L. (2024) Mechanisms of Antibiotic Resistance and Developments in Therapeutic Strategies to Combat Klebsiella pneumoniae Infection. Infect Drug Resist 17:1107-1119. http://doi.org/10.2147/IDR.S453025. PMID: 38525477; PMCID: PMC10960543.
[13] Sharafi T, Ghaemi EA, Rafiee M, Ardebili A. (2024) Combination antimicrobial therapy: in vitro synergistic effect of anti-staphylococcal drug oxacillin with antimicrobial peptide nisin against Staphylococcus epidermidis clinical isolates and Staphylococcus aureus biofilms. Ann Clin Microbiol Antimicrob 23(1):7. http://doi.org/10.1186/s12941-024-00667-6. PMID: 38245727; PMCID: PMC10800071.
[14] Torres Salazar BO, Heilbronner S, Peschel A, Krismer B. (2021) Secondary Metabolites Governing Microbiome Interaction of Staphylococcal Pathogens and Commensals. Microb Physiol 31(3):198-216. http://doi.org/10.1159/000517082. PMID: 34325424.
[15] Barbour A, Elebyary O, Fine N, Oveisi M, Glogauer M. (2022) Metabolites of the oral microbiome: important mediators of multikingdom interactions. FEMS Microbiol Rev 46(1):fuab039. http://doi.org/10.1093/femsre/fuab039. PMID: 34227664.
[16] Gasaly N, de Vos P, Hermoso MA. (2021) Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front Immunol 12:658354. http://doi.org/10.3389/fimmu.2021.658354. PMID: 34122415; PMCID: PMC8187770 .
[17] Pompilio A, Scocchi M, Mangoni ML, Shirooie S, Serio A, et al. (2023) Bioactive compounds: a goldmine for defining new strategies against pathogenic bacterial biofilms? Crit Rev Microbiol 49(1):117-149. http://doi.org/10.1080/1040841X.2022.2038082. PMID: 35313120.
Downloads
Published
Issue
Section
License

This work is licensed under a Creative Commons Attribution 4.0 International License.
All articles in the World Journal of Experimental Biosciences are published under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0) ( (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.